Concepts of ammonia/hydrogen engines for marine application (CAHEMA)
Project participants: Leilei Xu, Mark Treacy, Xue-Song Bai
Funding agency: Swedish Transport Administration (Trafikverket) via Nordic Maritime Transport and Energy Research Programme of Nordic Energy Research (NER).
Website of the Nordic Maritime Transport and Energy Research Programme of Nordic Energy Research
Purpose and goals
Maritime transport currently contributes approximately 3% of global greenhouse gas (GHG) emissions. It is responsible for carrying around 80% of global trade by volume. Trade volumes and GHG emissions are expected to rise further over the coming decades, unless significant action is taken. Therefore, in 2018, the IMO adopted the ‘Initial IMO Strategy on Reduction of GHG emissions from ships’ in order to help stabilise GHG emissions from shipping, and align itself with the goals of the Paris Agreement to limit global warming to an increase of 2 deg C. The IMO strategy aims to reduce GHG emissions from shipping by 50% by the year 2050, and phase them out before the end of the century, despite growing trade volumes.
To achieve this aim, drastic actions are needed. Current GHG emissions derive from the use of fossil fuels, primarily 'Heavy Fuel Oil', a type of cheap diesel fuel oil used onboard ships. To reduce GHG emissions, this project unites 2 of the world's leading marine engine manufacturers and shipping companies, and 5 leading universities from the Nordic countries to develop knowledge about the carbonless marine fuels hydrogen and ammonia. Replacing one fuel for another may appear trivial, but if current fuels were simply replaced by hydrogen or ammonia, ship engines would immediately come to a halt. Ammonia does not burn at all in ship engines, the way they are currently built. Hydrogen may burn, but it is very difficult to store onboard a ship, because it would have to be kept at temperatures that are so low that they do not naturally occur on earth. Luckily, hydrogen could be produced from ammonia, though this requires energy. A small amount of hydrogen could be produced from the waste heat of the exhaust gases of the engine, and this may just be enough to successfully burn and operate a marine engine.
The work in this project hypothesises that marine engines could use a combination of ammonia and hydrogen as fuels, based on new engine concepts, to operate successfully and without the emission of large amounts of pollutants and GHG. This hypothesis will have to be proven, by analysing the chemical reactions, and establishing computational models that can predict these reactions. Knowledge of the chemical reactions, will allow to simulate the physical processes taking place in an engine using computational models. Finally, the concepts developed herein will be proven with engine experiments, and their environmental impact will be assessed.
Sample results
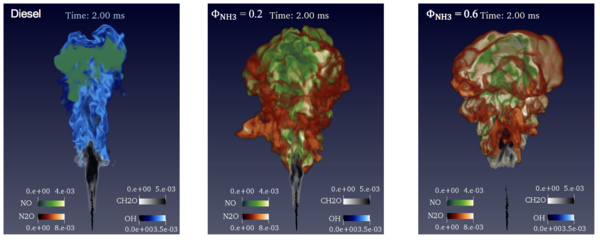
Large eddy simulation of ammonia/diesel RCCI combustion in a constant volume vessel
A high-fidelity numerical simulation, using the method known as large eddy simulation (LES), was carried out to investigate a marine engine concept, the so-called reactivity-controlled compression ignition (RCCI) engine concept. The aim was to study how ammonia is ignited by a diesel pilot flame and the formation of pollutant species NO and greenhouse gas N2O. Ammonia is a hydrogen carrier and carbonless in its molecule, and it has been considered a potential fuel for marine transport. Ammonia is difficult to burn in standard marine engines and novel combustion concepts, such as RCCI, may solve the problem. Figure 1 shows the LES prediction of four species in the flames: NO, N2O, CH2O (a product of low-temperature ignition of diesel), and OH (a high reactive radical species in the high temperature reaction zone of flame). It is clear that in the pure diesel flame the NO formation is in the downstream flame, while it is formed in the entire flame in the diesel/ammonia RCCI combustion. The ignition of diesel is delayed when injected into the ammonia/air mixture; the ignition delay time increases with increasing ammonia concentration in the ammonia/air mixture. This work reveals how the spray generated turbulent flow interacts with chemical reactions in the mixing layer of the diesel jet and the ambient ammonia/air mixture.
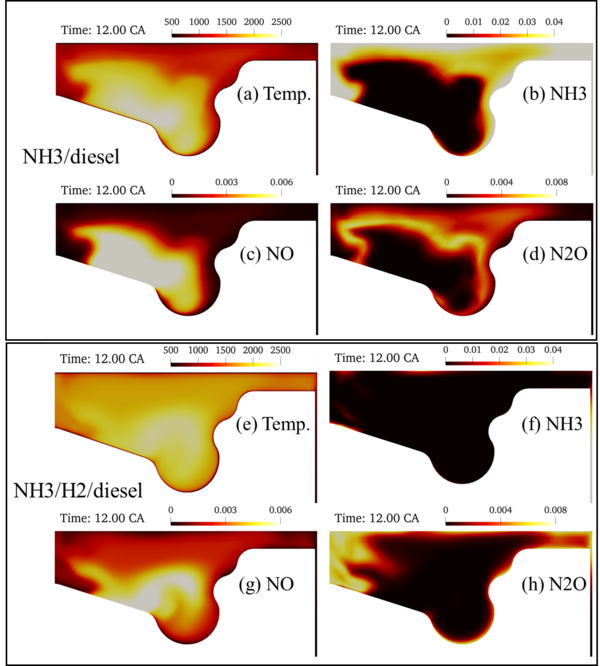
CFD study of ammonia/diesel RCCI combustion in a four-stroke heavy-duty engine
CFD simulations were carried out to investigate the concept of reactivity-controlled compression ignition (RCCI) engine in a four-stroke heavy-duty diesel engine. The simulation employed Reynolds averaged Navier-Stokes (RANS) turbulence models, and a reduced chemical kinetic mechanism. The engine was first filled with ammonia/air mixture, and then diesel was injected when the piston was close to the top-dead-centre (TDC). Different ammonia/diesel energy ratio (EP) was studied. Figure 2 shows the distribution of temperature, and mass fractions of unburned ammonia, NO and N2O in a cross-section of the engine cylinder. The ammonia/diesel energy ratio (EP) is 0.8. The engine was operating at part load with IMEP of 4 bars. It is shown that at this condition the ammonia combustion efficiency is rather low, with unburned ammonia near the walls and in the squish region. The addition of 10% (vol.%) hydrogen to the ammonia was found to significantly improve the combustion efficiency and reduce the unburned ammonia slip.